Carbon Dioxide Capture and Storage
Carbon Dioxide (CO2) capture and storage has the potential for significantly reducing the amount of CO2 released into the atmosphere. The technologies needed to separate CO2 from other gases and to sequester CO2 are known. Further development is needed to implement CO2 capture and storage on a large scale.
By burning fossil fuels—coal, oil, and natural gas—we are adding to the concentration of CO2, in the atmosphere. The CO2 concentration is now 379 parts per million. This is significantly more than it has been at any time in the past 600,000 years. The consensus among climatologists is that the increased CO2 concentration is the main cause of the increase in global temperature.
One way to reduce the amount of CO2 being released into the atmosphere is to rely more on alternative energy sources that do not produce CO2. These include hydroelectric, wind, solar, nuclear, geothermal, and tidal energy. Each of these has limitations, and it will be difficult to make a quick shift from fossil fuels to these other sources. But what if the CO2 produced by burning fossil fuels did not reach the atmosphere? Instead of letting CO2 go up the smokestack and into the air, can we capture it and put it somewhere? Is this possible?
Yes, it is. The process is called CO2 capture and storage. It is being done on a small scale right now. It has the potential to make a significant difference in the amount of CO2 we release into the atmosphere. As the name implies, there are two phases to the process. The first challenge is to capture the CO2 instead of letting it go up the smokestack. Then it has to be stored or “sequestered” safely and for a long time. The idea of sequestering CO2 to reduce the amount entering the atmosphere is fairly new. But the technology needed to do this has been developed for other reasons. We have a head start.
Capturing CO2
The best place to capture CO2 is at the major sources of emissions. Power stations that generate electricity produce about one-third of global CO2 emissions. In addition, CO2 is a by-product of iron and steel production, and cement production. CO2 is also removed from natural gas before it can be used as a fuel. These industrial processes are good candidates for CO2 capture and storage because they are large-scale sources in a fixed place. In contrast, it would be difficult to capture CO2 emissions from automobiles. The chief fossil fuels for power stations are natural gas and coal. These fuels are burned in the presence of air. The resulting heat is used to create steam that drives turbines, which turn electrical generators. Or, gas may be burned to drive turbines directly. In either case, the oxygen in the air combines with carbon in the fuel to produce CO2. The CO2 is released into the air. When natural gas is burned, hydrogen from methane (CH4) also combines with oxygen to form water.
But the air that was used to burn the fuel contained mostly nitrogen. This nitrogen does not participate in the combustion process. It is passed through and goes up the smokestack. The power-plant emissions, called flue gases, are typically only 10% to 15% CO2 in a coal-burning plant and about 5% when natural gas is the fuel. In principle we could store all the flue gases, but this would fill storage capacity mostly with nitrogen that does not need to be sequestered. For the CO2 to be stored efficiently, it first has to be separated from the other flue gases. How is this done?
There are three strategies:
- Separate the CO2 after combustion.
- Take the carbon out of the fuel before combustion so that we burn only hydrogen and produce only water.
- Burn fossil fuels in oxygen rather than air, resulting in concentrated CO2.
|
Click for animation showing separation of CO2 after combustion. |
Chemical solutions can be used to dissolve the CO2 while passing the other gases to the atmosphere. The technique most widely used today employs a group of compounds called amines. They absorb CO2 by forming chemical bonds, particularly when at high pressure and low temperature. This process is called “scrubbing.” The resulting chemical solution is later heated and the pressure reduced, releasing concentrated CO2.
Other solvents are used to dissolve CO2 without chemical bonding. In this physical absorption process the CO2 dissolves under pressure and is later removed from the solvent by reducing the pressure. The solvent may then be reused. Another strategy for capturing CO2 is to cool the flue gases to the point where the CO2 becomes liquid. This process requires considerable energy for refrigeration. An advantage is that the liquid can be easily transported by truck or ship. It is also possible to separate gases by using thin films called membranes. Some gases will pass through a membrane faster than others. This allows the different gases to be separated from one another.
Getting the Carbon Out before Combustion
|
Click for animation showing extraction of carbon before combustion. |
The fuel in natural gas is methane (CH4). When burned it produces both CO2 and water (H2O). If we can take out the carbon before combustion we will be left with hydrogen, which produces only water when burned. Doing this involves reacting the fuel with oxygen and/or steam to produce carbon monoxide (CO) and hydrogen. The carbon monoxide is then reacted with more steam to produce CO2 and more hydrogen.…Finally, the CO2 is separated and the hydrogen is used as fuel in a gas turbine.
Combustion with Oxygen rather than Air
|
Click for animation showing combustion with oxygen rather than air. |
Nitrogen makes up 78% of air. It remains mostly unchanged in the fuel combustion process. It is the main gas that dilutes CO2 in the flue gas mixture. If the fuel is burned in pure oxygen, rather than air, the CO2 concentration in flue gas can be increased to over 80%. This process makes it unnecessary to do further concentrating of the flue gas before sequestration of the CO2. The challenge is how to separate oxygen from the rest of the air, which is mainly nitrogen. The strategies are similar to those used to separate CO2. The air can be chilled so that the oxygen liquefies. Membranes that pass oxygen and nitrogen at different rates can cause the separation. There are also materials that absorb nitrogen, thus separating it from the oxygen. They may then be made to release the nitrogen and be reused.
Sequestration Technologies
Once concentrated CO2 has been captured, the next step is to store it somewhere. Here are some options.
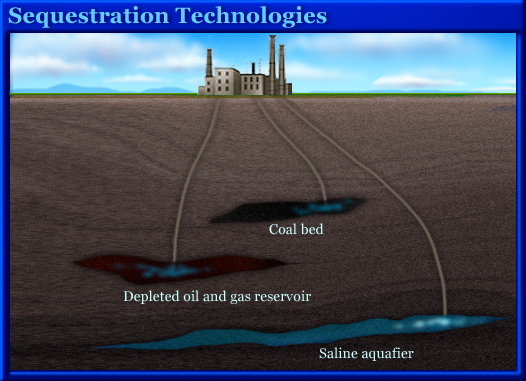
Geological Formations
Storage in geological formations is currently the most promising solution for widespread, long-term sequestration of CO2. Some projects are already under way. In order to reduce greenhouse gases and global warming, stored CO2 must be kept out of the atmosphere for hundreds or thousands of years. Oil and natural-gas reservoirs, deep saltwater aquifers, and coal seams have existed for millions of years with only very gradual changes. There is strong evidence that if properly managed, these formations could provide for long-term storage of CO2.
Depleted Oil and Natural-Gas Reservoirs
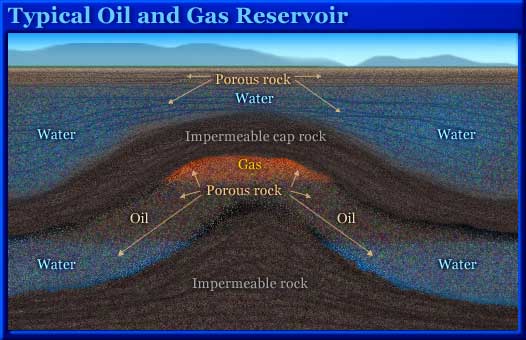
Many people believe that oil and natural gas are found in big underground caves. This is not the case. Rather, these hydrocarbons are in permeable and porous rocks such as sandstone. These rocks contain microscopic spaces, called pores, which fill with fluids. The fluids may be water, oil, or natural gas. An oil or natural-gas reservoir is more like a sponge than a bottle. Once an oil or natural-gas field has been productive for a period of time, a good portion of the hydrocarbons has been removed. There is space available to store CO2. The porous and permeable rock layer that contains these fluids is covered by an impermeable cap rock—often salt or shale—that does not let them pass through. Normally, oil and natural gas will tend to migrate upward through permeable rock because they are lighter than the water that is also found in such rock formations. The cap rock traps them. Since oil and natural gas have been sequestered in such formations for millions of years, there is good reason to believe that CO2 will remain there as well.
Enhanced Oil Recovery
Much of the technology needed to store CO2 in oil fields is already being used for a process known as enhanced oil recovery (EOR). When a reservoir is newly tapped, the oil is typically under pressure and flows freely to the surface. As oil is removed, pressure drops and pumping is needed to recover more. At some point recovery becomes uneconomical and is stopped, or additional techniques are used to extract more oil. One approach is to pump CO2 into the reservoir. This increases pressure so the oil flows more readily. Also, the CO2 dissolves in the oil and causes it to become less viscous and flow more easily. It expands in volume as well, further increasing pressure. CO2 is pumped into the reservoir through an "injection well." This forces the oil toward a "production well," where it rises to the surface.
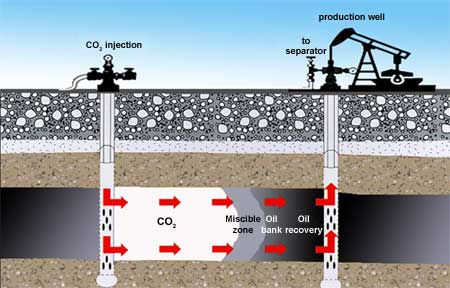 In enhanced oil recovery, CO2 is pumped into the reservoir through an injection well. It mixes with the remaining oil, forming a “miscible zone.” The pressure from the CO2 and expanding oil pushes an oil bank toward the production well, where it rises to the surface. Then the CO2 is separated from the oil and may be added to the stream of CO2 going into the injection well. A side effect of enhanced oil recovery is that the CO2 that was used to force oil out of the formation is now sequestered.
In enhanced oil recovery, CO2 is pumped into the reservoir through an injection well. It mixes with the remaining oil, forming a “miscible zone.” The pressure from the CO2 and expanding oil pushes an oil bank toward the production well, where it rises to the surface. Then the CO2 is separated from the oil and may be added to the stream of CO2 going into the injection well. A side effect of enhanced oil recovery is that the CO2 that was used to force oil out of the formation is now sequestered.
There are many EOR projects using CO2 injection around the world. A good example is the Weyburn field in Canada.
A side effect of enhanced oil recovery is that CO2 becomes sequestered in the rock formation. If the goal is CO2 storage rather than oil recovery, then depleted or nearly depleted oil fields may be used for sequestration even if they are not good candidates for enhanced oil recovery.
What are potential problems with CO2 sequestration? The big question is whether or not the CO2 will leak out of the reservoir. Leakage would be a problem because it would return the sequestered CO2 to the atmosphere. This would defeat the purpose of the project.
|
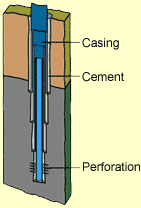
Well Casing |
When an oil well is drilled, the final step in the process is to insert steel pipe in the borehole and to fill the space between the outside of the pipe and the borehole with cement. The pipe, called a casing, is later perforated to allow oil to flow into the pipe and up to the surface. When the well is no longer productive, it may be sealed at the top.
If the leakage was sudden, it could kill people. CO2 is not poisonous, but if it substitutes for available oxygen, people can suffocate. Since CO2 is heavier than air, it can accumulate in low places such as cellars and valleys. There have been natural releases of CO2 that have caused death. One such disaster occurred at Lake Nyos in Cameroon. The lake is in a volcanically active area with carbon dioxide leaking into the waters of the lake from below. On August 21, 1986, there was a sudden release of CO2, which flowed into valleys around the lake and killed 1,800 people in nearby villages. There is no reason to expect CO2 sequestered in an old oil field to suddenly escape, but there are possibilities for slower leakage. Ironically, the wells themselves are a potential problem.
There may be hundreds of such wells in an oil field. If the field is old, the cement around the well casings may have deteriorated. This may provide a pathway for CO2 to escape. There are ways of recementing wells so that they provide a reliable seal for sequestered CO2.
Another possible pathway for CO2 to leak would be cracks in the cap rock. CO2 sequestration is proposed for regions that are geologically stable, where earthquakes are not likely to occur. However, the sequestering of CO2 itself could result in increased pressure under the cap rock that could result in cracks. The solution is to monitor pressure and take care not to exceed the limits of the formation.
Aquifers
There are many underground sealed geological "traps" that have never contained oil or natural gas. Their pores are filled with water. These are called aquifers. The aquifers that are most suitable for CO2 storage are deep underground. They are filled with salt water, so they are unsuitable for supplying or storing fresh water for human use. CO2 would partially dissolve in the water in the aquifer. In some rock types, it might react with minerals to form stable carbonate deposits. This would permanently lock up the CO2. Geological studies would need to be made, as is routinely done for oil and natural-gas reservoirs, to confirm that the aquifer would not leak CO2.
The world’s first CO2 injection program performed for climate-change considerations is offshore Norway in a deep saline aquifer in the North Sea known as the Sleipner field.
Coal Beds
Another potential storage medium is in coal deposits that are too deep to be mined. Coal is mostly carbon. It will absorb CO2 and lock it up permanently. Coal deposits usually contain methane. When CO2 is pumped into the coal, it is absorbed in preference to the methane, which is released. As with enhanced oil recovery, useful fuel is produced while sequestering CO2.
A problem with this approach is that coal swells when it absorbs CO2. This can result in reducing the pathways through which the gas flows. The result is more limited storage capacity.
Capture-and-Storage Case Studies
Two CO2 capture-and-storage projects currently under way are in the Sleipner field in the North Sea and in the Weyburn field in Canada.
This content has been re-published with permission from SEED. Copyright © 2025 Schlumberger Excellence in Education Development (SEED), Inc.