Water and Solubility
This activity created in partnership with AGI.
Water is a necessity in our lives. We use it every day in many different ways: to drink, wash, cook, and help get rid of waste. We provide plants with water so they can grow. We travel on water. In some parts of the world, water is used to generate electricity. And when there is not enough water in a particular area, living things suffer. Water is a very valuable substance.
Water takes many forms on the Earth: ocean, lake, river, stream, groundwater, ice, and water vapor in the atmosphere. All this water makes up Earth’s hydrosphere.
|
Video © AGI Note: When this video is finished, a different video will start automatically. To prevent this from happening, click the Stop button at the end of this video. |
Water has special properties that make it essential to life on Earth. Watch this video to learn about some of water’s properties and uses. Pay particular attention when the video discusses how water dissolves different substances. When the video is over, in your notebook write your ideas about why water is so important.
One of water’s most valuable properties is its ability to dissolve many substances. Without this property, many things on Earth would be different. For example, the oceans wouldn’t be salty. Plants and animals, which depend on moving dissolved substances into and out of their cells, would not exist.
|
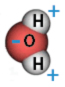
The water molecule has a positive charge on one side and a negative charge on the other. |
Water is made up of two hydrogen atoms bonded to one oxygen atom (H20). It is able to dissolve many materials because it is a polar, or charged molecule. This means that one side of the molecule (the hydrogen side) tends to be more positive than the other (the oxygen side), as shown in the diagram of the water molecule.
Our Experiment
In this activity, you will learn more about water’s role in dissolving some common substances. This is called solubility, a substance’s ability to be dissolved by water. You will also investigate how the solubility of substances might change if the water is at different temperatures.
Tools and materials
- Four transparent drinking cups, at least 250 mL (8.5 oz)
- Four substances for solubility testing:
- Vegetable oil
- Food coloring or ink
- Powdered iced tea, fruit drink mix, or colored sugar
- Sand or gravel
- Spoon or stick for stirring
- Water at room temperature
- Hot tap water
- Very cold water
- Notebook and pen
- Paper towels
- Alcohol thermometer (optional)
What to do
|
|
|
|
|
|
|
||
|
|
|
 |
Digging DeeperFind out more about: Water and Solubility. [2] |
|
|
|
This content has been re-published with permission from SEED. Copyright © 2025 Schlumberger Excellence in Education Development (SEED), Inc.
Course:
- Science [5]
- earth science [6]