Find out more: Water and Solubility
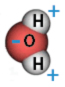 |
Image courtesy of U.S. Geological Survey.
|
A water molecule consists of two small atoms of hydrogen and one larger atom of oxygen (H2O). The two hydrogen atoms are bonded very strongly to the oxygen atom. The three atoms are not arranged in a straight line; instead, they form an angle with the hydrogen atom on one side of the molecule, as shown in the diagram.
The electrons that orbit around the three atoms are more strongly attracted to the oxygen atom than to the hydrogen atoms. Electrons have a negative electric charge. This gives the oxygen “side” of the water molecule a slightly negative electric charge. The hydrogen “side” of the water molecule has a slightly positive electric charge. Molecules like this, with one side positive and the other side negative, are called polar molecules.
The polar nature of water allows it to dissolve many substances; it is often called the “universal solvent” for this reason. Water acts as a solvent in two ways. First, it can dissolve a substance by forming hydrogen bonds with the substance’s molecules; water dissolves granulated sugar in this way. The second way is by water’s molecules surrounding and separating individual ions (charged particles) of a substance. Substances that dissolve in this second way (such as sodium chloride, or table salt) are called electrolytes. If you are interested, you can find solubility charts online that contain information about what materials do and do not dissolve in water.
Some substances, such as oil, do not dissolve in water. Although oil is a liquid, it is nonpolar. This means that it doesn’t have charged “sides” that the polar water molecule can attract. Oil is also less dense than water, so it floats on top. Sand, which is mostly made of silicon dioxide, has such a low solubility in water (0.12 g/L at 20˚C) that it appears not to dissolve at all.

