|
Nature of science: Assessing the ethics of scientific research—widespread use of nuclear fission for energy production would lead to a reduction in greenhouse gas emissions. Nuclear fission is the process taking place in the atomic bomb and nuclear fusion that in the hydrogen bomb. (4.5) |
|
|
Understandings: Nuclear fusion
Nuclear fission
|
International-mindedness:
Theory of knowledge:
Utilization: Aims:
|
|
Applications and skills: Nuclear fusion
Nuclear fission
Guidance:
|
|


 neutrons.
neutrons.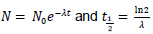 are given in section 1 of the data booklet.
are given in section 1 of the data booklet.