Understandings:
- The Arrhenius equation uses the temperature dependence of the rate constant to determine the activation energy.
- A graph of 1/T against ln k is a linear plot with gradient – Ea / R and intercept, lnA.
- The frequency factor (or pre-exponential factor) (A) takes into account the frequency of collisions with proper orientations.
Applications and skills:
- Analysing graphical representation of the Arrhenius equation in its linear form
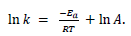
- Using the Arrhenius equation
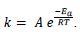
- Describing the relationships between temperature and rate constant; frequency factor and complexity of molecules colliding.
- Determining and evaluating values of activation energy and frequency factors from data.
Guidance:
- Use energy level diagrams to illustrate multi-step reactions showing the RDS in the diagram.
- Consider various data sources in using the linear expression in
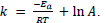 The expression ln The expression ln 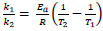 given in the data booklet. given in the data booklet.
|
Utilization:
- The flashing light of fireflies is produced by a chemical process involving enzymes.
- The relationship between the “lock and key” hypothesis of enzymes and the Arrhenius equation.
Syllabus and cross-curricular links:
Topic 6.1—collision theory
Aims:
- Aims 4 and 7: Use of simulations and virtual experiments to study effect of temperature and steric factors on rates of reaction.
- Aim 6: Experiments could include those involving the collection of temperature readings to obtain sufficient data for a graph.
- Aim 7: Graphing calculators can be employed to easily input and analyse data for Ea and frequency factor values.
|


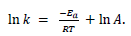
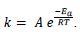
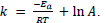 The expression ln
The expression ln 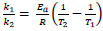 given in the data booklet.
given in the data booklet.