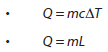|
Nature of science:
Evidence through experimentation: Scientists from the 17th and 18th centuries were working without the knowledge of atomic structure and sometimes developed theories that were later found to be incorrect, such as phlogiston and perpetual motion capabilities. Our current understanding relies on statistical mechanics providing a basis for our use and understanding of energy transfer in science. (1.8)
|
Understandings:
- Molecular theory of solids, liquids and gases
- Temperature and absolute temperature
- Internal energy
- Specific heat capacity
- Phase change
- Specific latent heat
Applications and skills:
- Describing temperature change in terms of internal energy
- Using Kelvin and Celsius temperature scales and converting between them
- Applying the calorimetric techniques of specific heat capacity or specific latent heat experimentally
- Describing phase change in terms of molecular behaviour
- Sketching and interpreting phase change graphs
- Calculating energy changes involving specific heat capacity and specific latent heat of fusion and vaporization
|
International-mindedness:
- The topic of thermal physics is a good example of the use of international systems of measurement that allow scientists to collaborate effectively
Theory of knowledge:
- Observation through sense perception plays a key role in making measurements. Does sense perception play different roles in different areas of knowledge?
Utilization:
- Pressure gauges, barometers and manometers are a good way to present aspects of this sub-topic
- Higher level students, especially those studying option B, can be shown links to thermodynamics (see Physics topic 9 and option sub-topic B.4)
- Particulate nature of matter (see Chemistry sub-topic 1.3) and measuring energy changes (see Chemistry sub-topic 5.1)
- Water (see Biology sub-topic 2.2)
|
Guidance:
- Internal energy is taken to be the total intermolecular potential energy + the total random kinetic energy of the molecules
- Phase change graphs may have axes of temperature versus time or temperature versus energy
- The effects of cooling should be understood qualitatively but cooling correction calculations are not required
Data booklet reference:
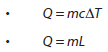
|
Aims:
- Aim 3: an understanding of thermal concepts is a fundamental aspect of many areas of science
- Aim 6: experiments could include (but are not limited to): transfer of energy due to temperature difference; calorimetric investigations; energy involved in phase changes
|