Spectroscopy
Spectroscopy is a branch of Physics and Chemistry that studies the interaction of light, or any electromagnetic radiation, such as radio-waves, with matter. Different waves carry different amounts of energy and lead to different interactions. Spectroscopy is a very powerful tool for detecting and analyzing molecules for a number of reasons. Spectroscopy
- Is sensitive and requires usually minute amounts of a substance in order to be able to identify it.
- Can be carried out on samples very far away, so it is used in astronomy.
- Is a mostly nondestructive method of analyzing things.
- Can yield detailed spatial and temporal information.
Actually, the eye is a spectroscopic "instrument" since it can detect differences in color, but scientific equipment can see very dim objects and can see the light in the minute details that we and animals cannot.
The Full Rainbow
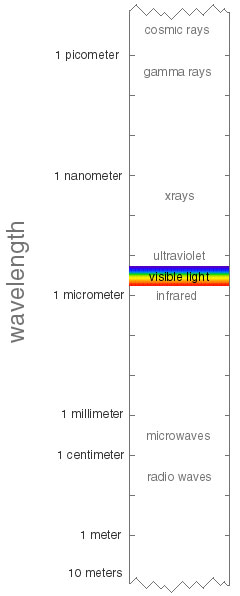 Electromagnetic radiation, of which visible light is one example, can be thought of as waves. Waves have a wavelength, which is inversely related to the frequency. The shorter the wavelength, the higher the frequency and the more energy is carried by the radiation. Energy is transmitted in small packets (quanta), also called photons. Let's tour the electromagnetic spectrum, starting with short wavelengths:
Electromagnetic radiation, of which visible light is one example, can be thought of as waves. Waves have a wavelength, which is inversely related to the frequency. The shorter the wavelength, the higher the frequency and the more energy is carried by the radiation. Energy is transmitted in small packets (quanta), also called photons. Let's tour the electromagnetic spectrum, starting with short wavelengths:
- Cosmic Rays (shorter than 1 pm)
- Gamma Rays (10 pm - 1 pm)
- X-rays (10 nm -- 1 nm)
- Ultraviolet (UV) (380 nm -- 10 nm)
- Visible Light (VIS) (red 780 nm -- violet 380 nm)
- Infrared (IR) (1 mm -- 1 micro m)
- Microwaves (1 cm -- 1 mm)
-
Radiowaves (longer than 1 cm)
As you can see, visible light is only a very small region in this spectrum.
Effects on Molecules
Molecules and atoms can interact with photons (electromagnetic waves) by absorbing their energy. This lowers the intensity of the radiation, which can be measured. This is called absorption spectroscopy. It is also possible that molecules and atoms are emitting photons, which can be detected - so called emission spectroscopy. There is a whole range of other techniques, which are discussed in specialised books.
The amount of energy a photon carries determines what effect it can have on molecules and atoms. Microwaves excite molecules to rotate, infrared light leads to vibrations of bonds within the molecule, visible and ultraviolet light excite electrons in bonds. Radiation with higher energy interacts with the core electrons near the nucleus of an atom or with the nucleus itself.
At very small wavelengths the energy of the 'light' is so great that it will break bonds and therefore destroy or modify chemicals. (This is how, for example, ultraviolet light has a bad effect on skin).
The relationship between wavelength and strength of absorption ("spectrum") can give important clues about the chemical structure of the molecule. That is how we know what kind of molecules are in interstellar gas clouds far away.
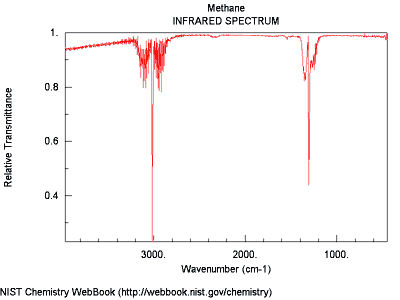 This image shows the spectrum of methane (CH4), a naturally occurring gas that is used for cooking and heating.
This image shows the spectrum of methane (CH4), a naturally occurring gas that is used for cooking and heating.
When the red line is near the top of the graph it indicates that methane is transmitting energy at that wavelength. When there is a dip toward the bottom it is because energy at that wavelength is being absorbed and therefore not "seen".
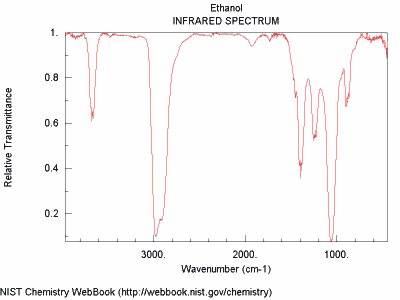 This is the infrared spectrum for ethanol (C2H5OH), a type of alcohol. The absorption pattern is different from that of methane. Using instruments that receive the infrared radiation transmitted from methane and ethanol would be able to identify these substances because of their unique patterns of transmitted and absorbed infrared radiation.
This is the infrared spectrum for ethanol (C2H5OH), a type of alcohol. The absorption pattern is different from that of methane. Using instruments that receive the infrared radiation transmitted from methane and ethanol would be able to identify these substances because of their unique patterns of transmitted and absorbed infrared radiation.
There is also a deep link between symmetry and spectroscopy, which states that the more symmetry a molecule has, the simpler its spectrum looks like. This was an important clue in the discovery of C60, which was predicted to have only four absorption lines in its infrared spectrum (a molecule of 60 atoms can have several hundred absorption lines).
This content has been re-published with permission from SEED. Copyright © 2025 Schlumberger Excellence in Education Development (SEED), Inc.

