How NMR Works
The motions of atomic nuclei can be directly controlled and detected by a nuclear magnetic resonance (NMR) apparatus.
Nuclear magnetic resonance - an impressive sounding name! But this is science, so the name wasn't just dreamed up to sound good. Let's look at the words:
Nuclear - Pertains to the nucleus of an atom, made up of protons and neutrons, or in the case of the hydrogen nucleus, made of one proton only.
magnetic resonanceA phenomenon by which a nucleus absorbs electromagnetic radiation of a specific frequency in the presence of a strong magnetic field. Isidor Isaac Rabi (1898 to 1988), an American physicist born in Austria, first detected magnetic resonance in 1938. Since then, magnetic resonance has been applied to the detection of light atoms (such as hydrogen in hydrocarbons) and has been used as a nondestructive way to study the human body. Synonyms: NMR, nuclear magnetic resonance from Schlumberger Oilfield Services Oilfield Glossary |
Magnetic - The nuclear motions are controlled with magnetic fields.
Resonance - We use resonance to efficiently manipulate the nuclei with magnetic fields.
The Earth and other spinning magnets
 Many (though not all) atomic nuclei can be thought of as very small bar magnets, with a north pole and a south pole. The nuclei spin at a constant rate, with the spin axis exactly coinciding with the line between the north and south poles.
Many (though not all) atomic nuclei can be thought of as very small bar magnets, with a north pole and a south pole. The nuclei spin at a constant rate, with the spin axis exactly coinciding with the line between the north and south poles.
Spinning bar magnets are actually pretty common in nature. Individual iron atoms, the earth, the sun, several planets and neutron stars are all spinning bar magnets. The earth is a more complicated spinning bar magnet than an atomic nucleus, because the geographical north pole (spin axis) of the earth does not exactly coincide with its magnetic north pole. Nuclei are better behaved: their magnetic and geographical poles coincide exactly.
The hydrogen nucleus, which consists of a single proton, is magnetic and an abundant component of water, gas and oil. We are particularly interested in these nuclei because we are looking for hydrocarbons.
Aligning the nuclear magnets

 Ordinarily, the north poles of nuclei point in whatever direction they want to point in. If no one tells them what to do, they will do what they want. The first step of an NMR measurement is to align the nuclear magnets with a strong magnetic field which we do by putting them in a large magnet. This makes them line up, with north poles of the nuclei pointing to the south pole of the magnet. Magnetic nuclei are happy when they are aligned by a magnetic field. They are in a comfortable state which physicists call equilibrium or low energy. It's a lot like a lazy kid just sitting on a playground swing, not going anywhere. He's happy.
Ordinarily, the north poles of nuclei point in whatever direction they want to point in. If no one tells them what to do, they will do what they want. The first step of an NMR measurement is to align the nuclear magnets with a strong magnetic field which we do by putting them in a large magnet. This makes them line up, with north poles of the nuclei pointing to the south pole of the magnet. Magnetic nuclei are happy when they are aligned by a magnetic field. They are in a comfortable state which physicists call equilibrium or low energy. It's a lot like a lazy kid just sitting on a playground swing, not going anywhere. He's happy.
|
Home project: Align the atomic nuclei in your hand. Materials needed: (1) a refrigerator magnet; (2) your hand. Procedure: Place magnet on hand. You've done it! Simple, but not very spectacular. |
Disturbing the magnets
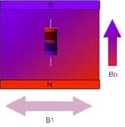
The second step of an NMR measurement is to get things moving. This is done by applying another magnetic field, but one in resonance with the nuclear motion.
 |
|
 |
|
An oscillating magnetic field called B1 is perpendicular to the field of the permanent magnet, B0. |
After a while the nuclei are tipped so that they are rotating in the plane that is perpendicular to the B0 field. |
It's like going over to the lazy kid on the swing and pushing him. But you don't just give him one big push. You give him many small pushes, every time he is near the top of the arc and going forward. A regular back-and-forth motion that can be amplified by small pushes is called a resonance. The same is true of nuclei. In order to get them to point away from the big magnet, you have to give them pushes.
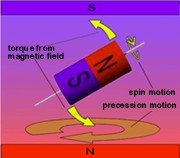 Because the nucleus is spinning, it behaves like a gyroscope or toy top. When a gyro or top is pointing straight up in the earth's gravitational field, it just spins. But if it tips away from straight up it goes into an orbital motion called precession. The precession speed, which is much slower than the spin speed, depends on the size and shape of the gyro, its spin speed and the strength of gravity.
Because the nucleus is spinning, it behaves like a gyroscope or toy top. When a gyro or top is pointing straight up in the earth's gravitational field, it just spins. But if it tips away from straight up it goes into an orbital motion called precession. The precession speed, which is much slower than the spin speed, depends on the size and shape of the gyro, its spin speed and the strength of gravity.
When a nucleus is tipped away from the direction of the strong magnetic field, it too precesses. The precession speed depends on the properties of the nucleus (rate of spin, etc.) and the strength of the magnetic field — very similar to the gyroscope. These properties never change, so all we need to know is the strength of the magnetic field to accurately predict the precession frequency. That's the frequency of pushes we must apply to get the nucleus tipped away from the main magnetic field and precessing. The pushes come from a second magnetic field which varies in time at the same rate as the precession - i.e. resonant with the nuclear motion. (Nuclear . . . magnetic . . . resonance — is it beginning to make sense?)
Once you get the lazy kid going on the swing, he will continue to swing for quite a while after you stop pushing him. Nuclei, too. All they need is a quick burst of radio waves, lasting maybe 10 microseconds (yes, microseconds) to keep them going for as much as several seconds (yes, seconds).
Monitoring the motion of the nuclei
| Did you know... What kind of magnetic field varies periodically to give the nuclei pushes at even intervals of time? Radio transmissions. And guess what, NMR instruments have the same kind of electronic circuits as radio stations. Some NMR equipment even uses the same frequency as FM radio, around 88 to 108 megahertz (88 to 108 million cycles per second). |
Even if you close your eyes, you know the lazy kid is still swinging. How? He is yelling his head off.
Once again, magnetic nuclei are pretty much the same. As long as they are out of alignment with the big magnet, or, in other words, out of equilibrium, they radiate radio waves. Each nucleus is like a very tiny radio station. And, sure enough, part of the NMR equipment is a radio receiver, to catch the emissions from the nuclei while they are moving. The first NMR apparatus was built with old World War II radar sets, which have both a radio transmitter and receiver in one unit.
Relaxation
Once you get the lazy kid going on the swing, he will continue to swing for quite a while after you stop pushing him. But he is not happy. He is out of equilibrium, in a high-energy state. That's just not his nature. After a while the swing will slow down for a variety of reasons — friction with the air and in the joints where the swing attaches to its supporting structure. But the kid, wanting to come to rest more quickly, drags his foot a little to slow himself down, until he is once again sitting still and happy.
The nucleus is a lot like the kid. You can get it moving with radio waves and it will keep going for quite a while even after you turn off the radio transmitter, but it is not happy. It will find a way to slowly return to equilibrium, oriented with the field of the permanent magnet in the NMR tool.
But wait a second. Nuclei don't have feet. So how do they slow down?
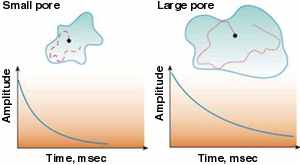 There are many ways for a nucleus to lose energy and return to equilibrium. One way, if the nucleus is in a liquid molecule, like water, is when it hits a solid surface. Every time the molecule hits a solid surface, the nucleus has a chance to return to happy alignment along the strong magnetic field. This is called . . . relaxation. You see, even nuclei like to relax.
There are many ways for a nucleus to lose energy and return to equilibrium. One way, if the nucleus is in a liquid molecule, like water, is when it hits a solid surface. Every time the molecule hits a solid surface, the nucleus has a chance to return to happy alignment along the strong magnetic field. This is called . . . relaxation. You see, even nuclei like to relax.
In larger pores the fluid molecules have more room to move around without bumping into the walls, so these collisions are less frequent. In a rock, NMR relaxation depends on the size of the pores: the larger the pores, the longer the NMR relaxation time.
The sensitivity of NMR to pore size has two simple, but very powerful, applications. The first is to permeability, which is determined by the size of its pores. More precisely, the permeability is proportional to the square of the diameter of the pores, so one expects the permeability to be proportional to the square of the NMR relaxation time. We have confirmed this relationship by doing laboratory tests on hundreds of different kinds of rocks.
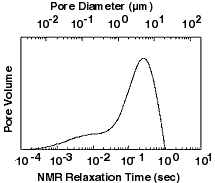 The second application of NMR data is to determine a distribution of pore sizes. Since pores within a single rock can vary greatly in size, the distributions are very broad. The pore size distribution tells geologists a lot about a rock - it is better than looking at it under a microscope.
The second application of NMR data is to determine a distribution of pore sizes. Since pores within a single rock can vary greatly in size, the distributions are very broad. The pore size distribution tells geologists a lot about a rock - it is better than looking at it under a microscope.
There's a lot more to it…
Alignment, disturbance, relaxation are the basics of NMR. But, like in all fields of science, this is just the start of a very big, very complicated and very interesting story. Several people have received Nobel Prizes for working out the details.
This content has been re-published with permission from SEED. Copyright © 2025 Schlumberger Excellence in Education Development (SEED), Inc.