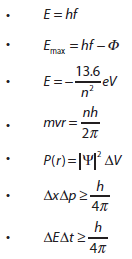|
Nature of science: Observations: Much of the work towards a quantum theory of atoms was guided by the need to explain the observed patterns in atomic spectra. The first quantum model of matter is the Bohr model for hydrogen. (1.8) Paradigm shift: The acceptance of the wave–particle duality paradox for light and particles required scientists in many fields to view research from new perspectives. (2.3) |
|
Understandings:
|
Theory of knowledge:
Utilization:
|
Applications and skills:
Guidance:
Data booklet reference:
|
Aims:
|