Understandings:
- Emission spectra are produced when photons are emitted from atoms as excited electrons return to a lower energy level.
- The line emission spectrum of hydrogen provides evidence for the existence of electrons in discrete energy levels, which converge at higher energies.
- The main energy level or shell is given an integer number, n, and can hold a maximum number of electrons, 2n2.
- A more detailed model of the atom describes the division of the main energy level into s, p, d and f sub-levels of successively higher energies.
- Sub-levels contain a fixed number of orbitals, regions of space where there is a high probability of finding an electron.
- Each orbital has a defined energy state for a given electronic configuration and chemical environment and can hold two electrons of opposite spin.
Applications and skills:
- Description of the relationship between colour, wavelength, frequency and energy across the electromagnetic spectrum.
- Distinction between a continuous spectrum and a line spectrum.
- Description of the emission spectrum of the hydrogen atom, including the relationships between the lines and energy transitions to the first, second and third energy levels.
- Recognition of the shape of an s atomic orbital and the px1, py and pz atomic orbitals.
- Application of the Aufbau principle, Hund’s rule and the Pauli exclusion principle to write electron configurations for atoms and ions up to Z = 36.
|
International-mindedness:
- The European Organization for Nuclear Research (CERN) is run by its European member states (20 states in 2013), with involvements from scientists from many other countries. It operates the world’s largest particle physics research centre, including particle accelerators and detectors used to study the fundamental constituents of matter.
Theory of knowledge:
- Heisenberg’s Uncertainty Principle states that there is a theoretical limit to the precision with which we can know the momentum and the position of a particle. What are the implications of this for the limits of human knowledge?
- “One aim of the physical sciences has been to give an exact picture of the material world. One achievement ... has been to prove that this aim is unattainable.” —Jacob Bronowski. What are the implications of this claim for the aspirations of natural sciences in particular and for knowledge in general?
Utilization:
- Absorption and emission spectra are widely used in astronomy to analyse light from stars.
- Atomic absorption spectroscopy is a very sensitive means of determining the presence and concentration of metallic elements.
|
Guidance:
- Details of the electromagnetic spectrum are given in the data booklet in section 3.
- The names of the different series in the hydrogen line emission spectrum are not required.
- Full electron configurations (eg 1s22s22p63s23p4) and condensed electron configurations (eg [Ne] 3s23p4) should be covered.
- Fireworks—emission spectra.
Orbital diagrams should be used to represent the character and relative energy of orbitals. Orbital diagrams refer to arrow-in-box diagrams, such as the one given below.
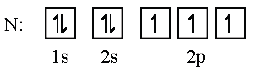
- The electron configurations of Cr and Cu as exceptions should be covered.
|
- Syllabus and cross-curricular links:
- Topics 3.1 and 3.2—periodicity
- Topic 4.1—deduction of formulae of ionic compounds
- Topic 6.1—Maxwell–Boltzmann distribution as a probability density function Physics topic 7.1 and option D.2—stellar characteristics
Aims:
- Aim 6: Emission spectra could be observed using discharge tubes of different gases and a spectroscope. Flame tests could be used to study spectra.
|


